Explain Why Water Has Different Properties From Carbon Dioxide
The carbon dioxide from the carbonated water dissolved in the indicator solution. Water but sometimes compounds like carbon dioxide skip the liquid phase altogether and transition directly to a solid when cooled.

Properties Kinds Of Molecules Relation Between Temperature Of Matter Its Physical State Science Online Molecules Online Science Human Body Education
Another important property of water is its ability to dissolve salts which significantly changes its density.

. The net effect is a partial dipole where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. After the reaction has taken place two new substances are formed from the reactants. A carbon dioxide molecule is a non polar molecule because of its straight line shape.
Chemical properties of carbon dioxide are generally related to its level of acidity. In carbon dioxide the carbon is. What type of covalent bond does carbon dioxide.
Water is a bent molecule due to the lone pairs present in oxygen. Carbon dioxide is soluble in water. Both these substances have different chemical properties from the compounds used as.
When it dissolves it forms a carbonic acid. Explain why there may be a difference in the rate of star jumps when breathing or not breathing and why doing star jumps without breathing is unsustainable. Most compounds show three phases when cooling at a constant room pressure ie.
At high pressures more carbon dioxide dissolves in water. Because of its dipole molecule water absorbs infrared radiation very efficiently. Carbon dioxide molecules cannot form hydrogen bonds with other carbon dioxide molecules.
On the other hand carbon dioxide is linear. Carbon dioxide is linear OCO polar bonds while water H-O-H is V-shaped polar bonds. Water is polar because it has a bent geometry that places the positively-charged hydrogen atoms on one side of the molecule and the negatively-charged oxygen atom on the other side of the molecule.
Thus due to this non-polarity of a molecule the physical properties like the boiling point of water molecule will reduce. Carbonated water also known as soda water sparkling water fizzy water club soda water with gas in many places as mineral water or especially in the US as seltzer or seltzer water is water containing dissolved carbon dioxide gas either artificially injected under pressure or occurring due to natural geological processes. How might this explain why carbon dioxide and water have such different properties.
Why is water a polar molecule wherelse carbon dioxide is not. Both water and carbon dioxide are triatomic molecules with similar formulas. Both water and carbon dioxide are triatomic molecules with similar formulas.
Electrochemical cells are constructed using different electrodes in Fe 2 Fe 3. The shapes of molecules have important implications. Because of its bent shape.
One is that even though both CO bonds and O-H bonds have dipoles carbon dioxide molecules are non-polar due to their linear shape and water molecules are polar due to their bent shape. Water forms a liquid instead of a gas because oxygen is more electronegative than the surrounding elements with the exception of fluorine. The products produced from autotrophs reacting water and carbon dioxide are oxygen and glucose.
Carbon dioxide cannot form hydrogen bonds with other species of molecules. Oxygen attracts electrons much more strongly than does hydrogen resulting in a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. Because carbon tetrachloride CCL is not soluble in water a test tube containing the two liquids will form two layers.
Start your trial now. As a result it approximately doubles the warming originally caused by carbon dioxide. Sulfur dioxide SO2 and carbon dioxide CO2 have somewhat different properties in spite of the fact that both are triatomic molecules with two terminal oxygen atoms.
This process is performed in laboratories or industrial facilities because of the highly specialized nature of this work. Tell students that they have seen carbon dioxide gas from your breath and carbon dioxide gas from carbonated water turn an indicator solution. Carbon dioxides structure and charge distribution make it hydrophobic.
The fact that water vapor is the dominant absorber in the Earths greenhouse effect can lead to a flawed narrative that anthropogenic carbon dioxide CO2 is. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen but it is polar enough that carbon dioxide can dissolve in water. Carbonic acid is what gives fizzy drinks their bubbles.
While most carbon dioxide units are slightly acidic by nature the level of acidity can be modified by dissolving the molecules in water. Can you explain why they are as different as they are. Describe how the properties of oxygen carbon dioxide and water contribute to their physiological functions.
Carbonation causes small bubbles to form giving the. This is because the pressure at normal atmosphere is too low for carbon dioxide to condense to a liquid. The molecules of carbon dioxide reacted with the water forming carbonic acid and changed the color of the indicator.
Explain why one of these is polar and the other is nonpolar. In contrast a water molecule is a polar molecule. The angle between bonds in water is 1045 making water a bent molecule.
The hydrophobic effect or the exclusion of compounds containing carbon and hydrogen nonpolar compounds is another unique property of water caused by the hydrogen bonds. The unique physical properties including a high heat of vaporization strong surface tension high specific heat and nearly universal solvent properties of water are also due to hydrogen bonding. The average salinity of the ocean is 347 parts per thousand.
Therefore if water molecule was linear like carbon dioxide molecule it would become nonpolar as the equal and opposite dipoles cancel out each other and the boiling point will be lower than 100 C o.
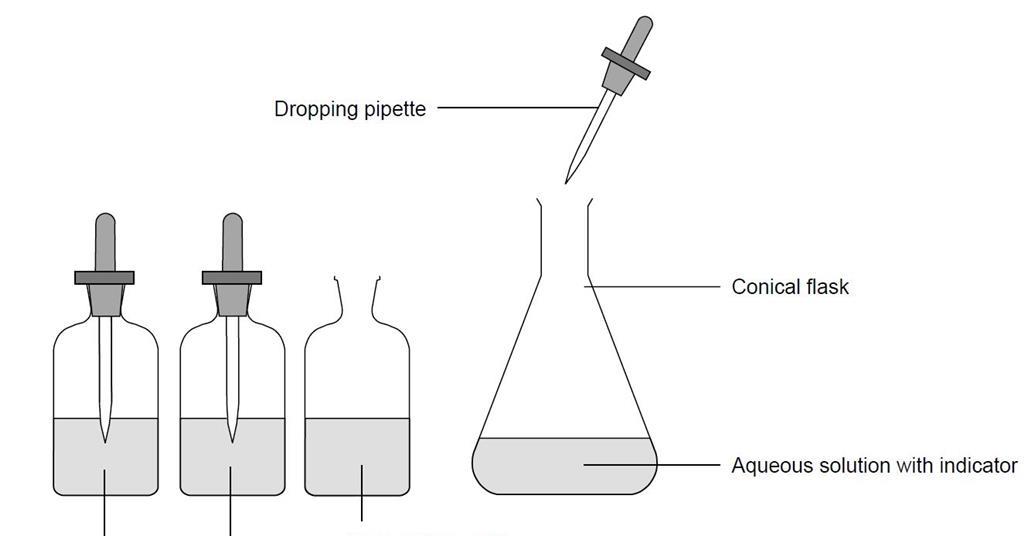
The Reaction Of Carbon Dioxide With Water Experiment Rsc Education

0 Response to "Explain Why Water Has Different Properties From Carbon Dioxide"
Post a Comment